Example:- air ,water, hydrogen, gold, wood, alcohol, petrol, carbon, rocks and minerals are all different kinds of matter because all of them occupy space and have mass.
- The only condition for something to be matter are that it should occupy space and have mass.
- The things like love friendship hurted good manner taste and smell etc do not occupy space and do not have mass. So it is not matter.
- Ancient Indian philosopher said that all the matter living or nonliving was made up of five basic elements air, water, fire, sky and earth.
- Modern day scientists classify matter into two ways on the basis of its physical properties and on the basis of its chemical properties.
- On the basis of physical properties of matter is classified as solid, liquid and gas.
- On the basis of chemical properties of matter is classified as elements, compounds and mixtures.
- Everything around us is made of tiny pieces or particle.
- Example:- our table is made of particle. our body is made of particles. Our book is made of a particles.
- The particle which make up matter are atoms or molecules.
Evidence for particles in matter:- the evidence for the existence of particles in matter and their motion comes from the experiments on diffusion and Brownian motion.
- Some experiments like dissolving of a solid in a liquid mixing of two gases and movement of pollen grain in water which can only be explained by zooming that all matter is made up of particles which are constantly moving.
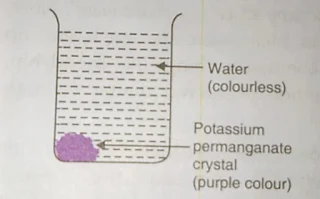
1- Dissolving a solid in a liquid:- when a crystal of potassium per magnet is placed in a beaker of water, the water slowly turns purple on its own even without steering both potassium permagnet crystal and water are made up of particles. the particles of potassium per magnet are purple coloured where as the particles of water are colourless. when the potassium permagnet crystal is put in water its particle separate from one another, actually on dissolving the particles of potassium per magnet get into the space between the particles of the water.
2- Mixing of two gases;- air is a colourless, gas when a gas jar is empty, it is actually filled with air bromine is a Red Brown liquid it forms vapour easily. A gas jar containing air is placed upside down on a gas Jar of bromine vapour or gas. we will see that the Red Brown vapours of bromine from the lower gas Jar sprayed of into air in the upper gas jar and after sometime the gas jar containing air also becomes completely Red Brown in colour. we can explained as follows: both air and bromine vapour are made of moving particle, the moving particles of bromine vapour and air collide with each other and bounce about in all directions due to which they get mixed uniformly.
Conclusion:- the matter is made up of tiny particles and the particles of matter are constantly moving.
3- Movement of pollen grains in water:- Robert Brown in 1827 performed this experiment in which, he suspended extremely small pollen grains in water on Looking through the microscope it was found that the pollen grains were moving rapidly throughout water in a zigzag way it was also observed that the water faster the pollen grains move on the surface of water.He concluded that the pollen grains move on the surface of water because they are constantly being hit by the fast moving particles of water.
Conclusion:- from the existence of Brownian motion gives us two conclusion that matter is made up of tiny particles and that the particles of matter are constantly moving.









0 Comments